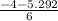Chemistry, 19.02.2020 00:42 sotoamerica0814
For the equilibrium Br2(g) + Cl2(g) ⇌ 2 BrCl(g) the equilibrium constant Kp is 7.0 at 400 K. If a cylinder is charged with BrCl(g) at an initial pressure of 1.00 atm and the system is allowed to come to equilibrium what is the final (equilibrium) pressure of BrCl? For the equilibrium the equilibrium constant is 7.0 at 400 . If a cylinder is charged with at an initial pressure of 1.00 and the system is allowed to come to equilibrium what is the final (equilibrium) pressure of BrCl?
a. 0.31 atm b. 0.15 atmc. 0.57 atmd. 0.22 atme. 0.45 atm

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 23.06.2019 09:30
Organisms that live in the alpine and taiga biomes have developed unique adaptations that aid in their survival. moss campion is one of the plants found in the alpine biome. it has small leaves and a cushion shape that protect it from the wind and freezing temperatures in the alpine. how has the moss campion adapted to enable its survival in the alpine biome? a. waxy needles b. cone-shaped c. thin trunks d. low-growing
Answers: 1

Chemistry, 23.06.2019 13:50
Use the periodic table and your knowledge of isotopes to complete these statements. when polonium-210 emits an alpha particle, the child isotope has an atomic mass of 1-131 undergoes beta-minus decay. the chemical symbol for the new element is fluorine-18 undergoes beta-plus decay. the child isotope has an atomic mass of done intro donne
Answers: 1

Chemistry, 23.06.2019 14:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 6.00 mol fe and 8.45 mol nio(oh) react?
Answers: 1
You know the right answer?
For the equilibrium Br2(g) + Cl2(g) ⇌ 2 BrCl(g) the equilibrium constant Kp is 7.0 at 400 K. If a cy...
Questions















Computers and Technology, 02.12.2019 20:31





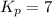
![K_p= \frac{[BrCl_2]^2}{[Br_2][Cl_2]}](/tpl/images/0514/9838/34fde.png)
![7 = \frac{[1-2x]^2}{[x][x]}](/tpl/images/0514/9838/d691d.png)
![7 = \frac{[1-2x]^2}{[x]^2}](/tpl/images/0514/9838/b5b46.png)
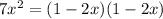
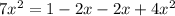
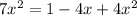
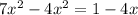
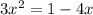
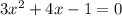 ----------(quadratic equation)
----------(quadratic equation)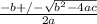
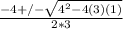
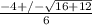
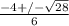
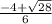 or
or 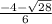
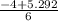 or
or