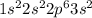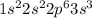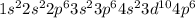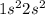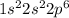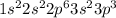Chemistry, 19.02.2020 00:22 gracebrownnn
Group the following electron configurations in pairs that would represent similar chemical properties at their atoms;
a. 1s2 2s2 2p6 3s2
b. 1s2 2s2 2p3
c. 1s2 2s2 2p6 3s2 3p6 4s2 3d104p6
d. 1s22s2
e. 1s2 2s2 2p6
f. 1s2 2s2 2p6 3s2 3p3

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
Group the following electron configurations in pairs that would represent similar chemical propertie...
Questions

Mathematics, 10.11.2019 21:31


Mathematics, 10.11.2019 21:31

Mathematics, 10.11.2019 21:31


Mathematics, 10.11.2019 21:31


Mathematics, 10.11.2019 21:31

History, 10.11.2019 21:31




Chemistry, 10.11.2019 21:31



Mathematics, 10.11.2019 21:31

History, 10.11.2019 21:31



Mathematics, 10.11.2019 21:31