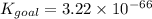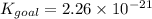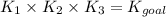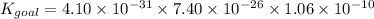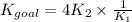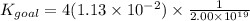Chemistry, 18.02.2020 23:57 briannaleiigh
Determine the value of the equilibrium constant, Kgoal, for the reaction
N2(g)+H2O(g)⇌NO(g)+12N2H4(g), Kgoal=?
by making use of the following information:
1. N2(g)+O2(g)⇌2NO(g), K1 = 4.10×10−31
2. N2(g)+2H2(g)⇌N2H4(g), K2 = 7.40×10−26
3. 2H2O(g)⇌2H2(g)+O2(g), K3 = 1.06×10−10
Express your answer numerically.
Kgoal =
Part B
Determine the equilibrium constant, Kgoal, for the reaction
4PCl5(g)⇌P4(s)+10Cl2(g), Kgoal=?
by making use of the following information:
P4(s)+6Cl2(g)⇌4PCl3(g), K1=2.00×1019
PCl5(g)⇌PCl3(g)+Cl2(g), K2=1.13×10−2
Express your answer numerically.
Kgoal =

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1


Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
Determine the value of the equilibrium constant, Kgoal, for the reaction
N2(g)+H2O(g)⇌NO...
N2(g)+H2O(g)⇌NO...
Questions













History, 14.06.2020 04:57




Mathematics, 14.06.2020 04:57