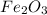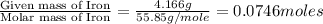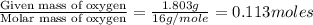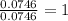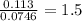Chemistry, 18.02.2020 23:31 navleen4owjrk1
In a reaction involving iron, Fe, and oxygen, O. it was determined that 4.166 grams of iron reacted with 1.803 grams of oxygen. From this information, determine the empirical formula of the compound that resulted. a. FEO2b. FeO3c. Fe2Od. Fe2O3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1

Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
You know the right answer?
In a reaction involving iron, Fe, and oxygen, O. it was determined that 4.166 grams of iron reacted...
Questions

Chemistry, 10.11.2020 08:20



Biology, 10.11.2020 08:20


Biology, 10.11.2020 08:20

Mathematics, 10.11.2020 08:20

Biology, 10.11.2020 08:20

Social Studies, 10.11.2020 08:20

English, 10.11.2020 08:20


Mathematics, 10.11.2020 08:20


Computers and Technology, 10.11.2020 08:20


History, 10.11.2020 08:20


Social Studies, 10.11.2020 08:20

Mathematics, 10.11.2020 08:20

Chemistry, 10.11.2020 08:20