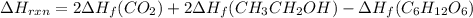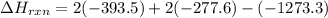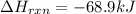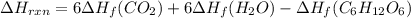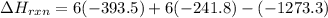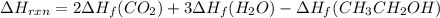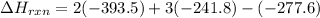Be sure to answer all parts. In winemaking, the sugars in grapes undergo fermentation by yeast to yield CH3CH2OH (ethanol) and CO2. During cellular respiration, sugar and ethanol are "burned" to water vapor and CO2.
a. Using C6H12O6 for sugar, calculate ΔH o rxn of fermentation and of respiration (combustion). Fermentation = kJ Respiration = kJ
b. Write a combustion reaction for ethanol. Include the physical states of each reactant and product. Which releases more heat from combustion per mole of C, sugar or ethanol? sugar ethanol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
Be sure to answer all parts. In winemaking, the sugars in grapes undergo fermentation by yeast to yi...
Questions




Mathematics, 19.12.2019 02:31






Mathematics, 19.12.2019 02:31










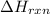 can be calculated using the standard enthalpy of formation (ΔHf) of products and reactants as follows:
can be calculated using the standard enthalpy of formation (ΔHf) of products and reactants as follows: 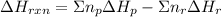 (1)
(1)