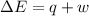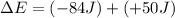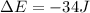Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Suppose a system receives a ""deposit"" of 50 J of work from the surroundings and loses a ""withdraw...
Questions


Mathematics, 28.08.2019 07:30


Biology, 28.08.2019 07:30



Biology, 28.08.2019 07:30

Mathematics, 28.08.2019 07:30

History, 28.08.2019 07:30


Mathematics, 28.08.2019 07:30



Mathematics, 28.08.2019 07:30

Physics, 28.08.2019 07:30



Computers and Technology, 28.08.2019 07:30

Mathematics, 28.08.2019 07:30

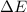 for this process is, -34 J
for this process is, -34 J