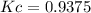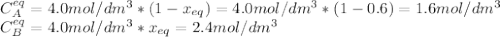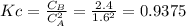The elementary reversible reaction
2A <-->B
is carried out in a flow re...

Chemistry, 18.02.2020 20:16 crispingolfer7082
The elementary reversible reaction
2A <-->B
is carried out in a flow reactor where pure A is fed at a concentration of 4.0 mol/dm 3 . If the equilib-rium conversion is found to be 60%,
(a) What is the equilibrium constant, KC if the reaction is a gas phase reaction?
(b) What is the KC if the reaction is a liquid-phase reaction?
(c) Write - rA solely as a function of conversion (i. e., evaluating all symbols) when the reaction isan elementary, reversible, gas-phase, isothermal reaction with no pressure drop with kA = 2 dm6 /mol•s and KC = 0.5 all in proper units.
(d) Repeat (c) for a constant-volume batch reactor.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

You know the right answer?
Questions


Mathematics, 30.06.2019 14:30




Social Studies, 30.06.2019 14:30

Mathematics, 30.06.2019 14:30


Mathematics, 30.06.2019 14:30

Health, 30.06.2019 14:30



Mathematics, 30.06.2019 14:30

Mathematics, 30.06.2019 14:30





Physics, 30.06.2019 14:30