
The rate law for the reaction
2NO(g) + Cl2(g) → 2NOCl(g)
is given by R = k[NO][Cl2]
If the following is the mechanism for the reaction, NO(g) + Cl2(g) → NOCl2(g) NOCl2(g) + NO(g) → 2NOCl(g)
which of the following statements accurately describes this reaction? Check all that apply.
-2nd order reaction
-The first step is the slow step.
-Doubling [NO] would quadruple the rate.
-Cutting [Cl2] in half would decrease the rate by a factor of two.
-The molecularity of the first step is 1.
-Both steps are termolecular.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1
You know the right answer?
The rate law for the reaction
2NO(g) + Cl2(g) → 2NOCl(g)
is given by R = k...
2NO(g) + Cl2(g) → 2NOCl(g)
is given by R = k...
Questions


Mathematics, 03.07.2019 22:00


English, 03.07.2019 22:00


History, 03.07.2019 22:00




Chemistry, 03.07.2019 22:00



Mathematics, 03.07.2019 22:00



Biology, 03.07.2019 22:00



Chemistry, 03.07.2019 22:00

Biology, 03.07.2019 22:00

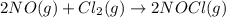
![k[NO][Cl_{2}]](/tpl/images/0514/1761/8cd0b.png)
 . Since, power of the concentrations of each of these is equal to 1. Therefore, order of the reaction is equal to 1 + 1 = 2.
. Since, power of the concentrations of each of these is equal to 1. Therefore, order of the reaction is equal to 1 + 1 = 2.

