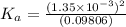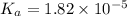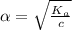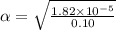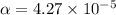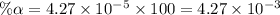Chemistry, 18.02.2020 05:22 Mangolinux7173
Start with 100.00 mL of 0.10 M acetic acid, CH3COOH. The solution has a pH of 2.87 at 25 oC. a) Calculate the Ka of acetic acid at 25 oC. b) Determine the percent dissociation for the solution.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

You know the right answer?
Start with 100.00 mL of 0.10 M acetic acid, CH3COOH. The solution has a pH of 2.87 at 25 oC. a) Calc...
Questions

History, 25.09.2019 10:00

Physics, 25.09.2019 10:00

Mathematics, 25.09.2019 10:00


Mathematics, 25.09.2019 10:00

Health, 25.09.2019 10:00



Social Studies, 25.09.2019 10:00


Mathematics, 25.09.2019 10:00


Mathematics, 25.09.2019 10:00

History, 25.09.2019 10:00

Mathematics, 25.09.2019 10:00

Mathematics, 25.09.2019 10:00

Chemistry, 25.09.2019 10:00


Biology, 25.09.2019 10:00

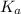 of acetic acid at
of acetic acid at 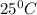 is
is 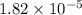
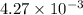
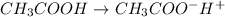
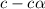
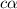
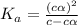
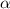 = ?
= ?![pH=-log[H^+]](/tpl/images/0513/9443/15713.png)
![2.87=-log[H^+]](/tpl/images/0513/9443/3a07c.png)
![[H^+]=1.35\times 10^{-3}M](/tpl/images/0513/9443/01bcb.png)
![[CH_3COO^-]=1.35\times 10^{-3}M](/tpl/images/0513/9443/0a4ad.png)
![[CH_3COOH]=(0.10M-1.35\times 10^{-3}=0.09806M](/tpl/images/0513/9443/5420f.png)