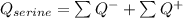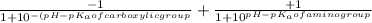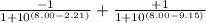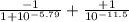Chemistry, 18.02.2020 02:32 peanutpinkypiepdma46
The p K a of the α‑carboxyl group of serine is 2.21 , and the p K a of its α‑amino group is 9.15 . Calculate the average net charge on serine if it is in a solution that has a pH of 8.80 .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2
You know the right answer?
The p K a of the α‑carboxyl group of serine is 2.21 , and the p K a of its α‑amino group is 9.15 . C...
Questions



Mathematics, 28.08.2019 21:00


Biology, 28.08.2019 21:00




English, 28.08.2019 21:00




Mathematics, 28.08.2019 21:00


Social Studies, 28.08.2019 21:00



English, 28.08.2019 21:00

Social Studies, 28.08.2019 21:00

Computers and Technology, 28.08.2019 21:00

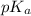 of carboxylic group of serine = 2.21
of carboxylic group of serine = 2.21