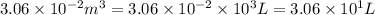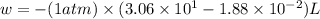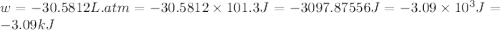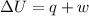Chemistry, 18.02.2020 02:18 emily12403
At 373.15K and 1 atm, the molar volume of liquid water and steamare 1.88 X 10-5 m3 and 3.06 X 10-2m3, respectively. Given that the heat of vaporization ofwater is 40.79 kJ/mol, calculate the values of ?H and ?Ufor 1 mole in the following process:
H2O (l, 373.15 K, 1 atm) ---> H2O(g, 373.15 K, 1 atm)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1
You know the right answer?
At 373.15K and 1 atm, the molar volume of liquid water and steamare 1.88 X 10-5 m3 and 3.06 X 10-2m3...
Questions

Mathematics, 15.12.2020 18:00

English, 15.12.2020 18:00

Mathematics, 15.12.2020 18:00




Mathematics, 15.12.2020 18:00

Mathematics, 15.12.2020 18:00

English, 15.12.2020 18:00

Computers and Technology, 15.12.2020 18:00


Mathematics, 15.12.2020 18:00

Chemistry, 15.12.2020 18:00

Arts, 15.12.2020 18:00

Mathematics, 15.12.2020 18:00


English, 15.12.2020 18:00

Mathematics, 15.12.2020 18:00


Mathematics, 15.12.2020 18:00

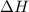 and
and 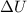 is, 40.79 kJ and 37.7 kJ respectively.
is, 40.79 kJ and 37.7 kJ respectively.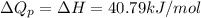
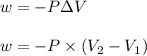
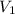 = initial volume =
= initial volume = 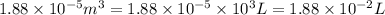
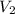 = final volume =
= final volume =