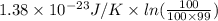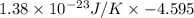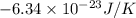Chemistry, 18.02.2020 02:03 kayranicole1
A chlorine Cl and bromine Br atom are adsorbed on a small patch of surface (see sketch at right). This patch is known to contain 100 possible adsorption sites. The Cl and bromine Br atoms have enough energy to move from site to site, so they could be on any two of them. Suppose the Br atom desorbs from the surface and drifts away. Calculate the change in entropy. Round your answer to 2 significant digits, and be sure it has the correct unit symbol.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
A chlorine Cl and bromine Br atom are adsorbed on a small patch of surface (see sketch at right). Th...
Questions


Social Studies, 01.09.2019 21:00







Computers and Technology, 01.09.2019 21:00

History, 01.09.2019 21:00


Mathematics, 01.09.2019 21:00


English, 01.09.2019 21:00

History, 01.09.2019 21:00


Spanish, 01.09.2019 21:00

Social Studies, 01.09.2019 21:00


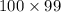
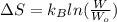
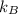 = Boltzmann constant =
= Boltzmann constant = 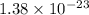
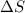 = change in entropy
= change in entropy