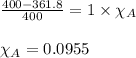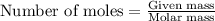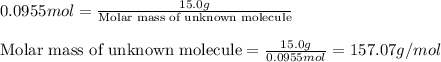Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 23.06.2019 10:10
In a covalent bond, two atoms are held together by the attraction between . the number of covalent bonds that an atom can form depends on the number of in the atom.
Answers: 2

Chemistry, 23.06.2019 17:10
Which substance produces hydroxide ions in solution? a. an arrhenius acid b. an arrhenius base c. a brønsted-lowry acid d. a brønsted-lowry base e. an amphoteric substance
Answers: 3

Chemistry, 23.06.2019 18:50
Why are very high temperatures and pressures required for fusion to occur? to generate the neutrons that are needed to break the nuclei o to overcome the repulsion between the protons in the nuclei that join to maintain the proper conditions to keep the chain reaction going to keep the uranium fuel separate from the control rods
Answers: 1
You know the right answer?
At 39.5 o C, the vapor pressure of pure acetone (MM = 58.08 g/mol) is 400.0 torr. If 15.0 grams of a...
Questions

Business, 05.10.2021 22:40


History, 05.10.2021 22:40




Chemistry, 05.10.2021 22:40

Mathematics, 05.10.2021 22:40

Mathematics, 05.10.2021 22:40

Business, 05.10.2021 22:40

Chemistry, 05.10.2021 22:40


SAT, 05.10.2021 22:40

Mathematics, 05.10.2021 22:40


Business, 05.10.2021 22:40



Chemistry, 05.10.2021 22:40

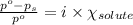
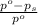 = relative lowering in vapor pressure
= relative lowering in vapor pressure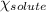 = mole fraction of solute = ?
= mole fraction of solute = ?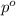 = vapor pressure of pure acetone = 400 torr
= vapor pressure of pure acetone = 400 torr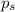 = vapor pressure of solution = 361.8 torr
= vapor pressure of solution = 361.8 torr