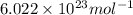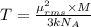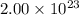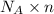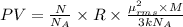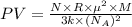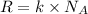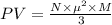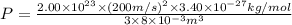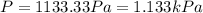Chemistry, 17.02.2020 23:47 YannahRussell
A cubic box with sides of 20.0 cm contains 2.00 × 1023 molecules of helium with a root-mean-square speed (thermal speed) of 200 m/s. The mass of a helium molecule is 3.40 × 10-27 kg. What is the average pressure exerted by the molecules on the walls of the container? (The Boltzmann constant is 1.38 × 10-23 J/K and the ideal gas constant is R = 8.314 J/mol•K .) (12 pts.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1


Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
A cubic box with sides of 20.0 cm contains 2.00 × 1023 molecules of helium with a root-mean-square s...
Questions



Physics, 29.10.2021 07:00


Mathematics, 29.10.2021 07:00




Mathematics, 29.10.2021 07:00


English, 29.10.2021 07:00


Physics, 29.10.2021 07:00

Social Studies, 29.10.2021 07:00

Geography, 29.10.2021 07:00

History, 29.10.2021 07:00



Mathematics, 29.10.2021 07:10

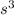
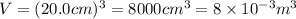
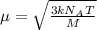
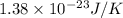
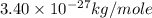
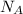 = Avogadro’s number =
= Avogadro’s number =