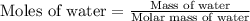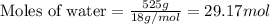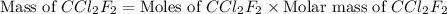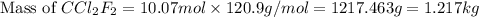Chemistry, 17.02.2020 20:20 therealpr1metime45
A particular refrigerant cools by evaporating liquefied CCl 2F 2. How many kg of the liquid must be evaporated to freeze a tray of water to ice (at zero degrees C)? The tray contains 525 grams water. Molar heat of fusion of ice = 6.01 kJ/mol. Molar heat of vaporization of CCl 2F 2 = 17.4 kJ/mole

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

You know the right answer?
A particular refrigerant cools by evaporating liquefied CCl 2F 2. How many kg of the liquid must be...
Questions

Spanish, 05.10.2021 14:00


Mathematics, 05.10.2021 14:00

History, 05.10.2021 14:00



SAT, 05.10.2021 14:00


Medicine, 05.10.2021 14:00



History, 05.10.2021 14:00

SAT, 05.10.2021 14:00

Mathematics, 05.10.2021 14:00

English, 05.10.2021 14:00

Mathematics, 05.10.2021 14:00


Mathematics, 05.10.2021 14:00

English, 05.10.2021 14:00

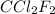 evaporated must be, 1.217 kg
evaporated must be, 1.217 kg