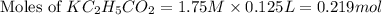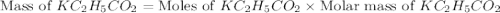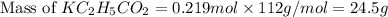Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1


Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

You know the right answer?
A chemistry graduate student is given 125. mL of a 1.30 M propanoic acid (HC2H, Co2) solution. Propa...
Questions

Mathematics, 29.03.2020 18:53

Mathematics, 29.03.2020 18:53







English, 29.03.2020 18:53



History, 29.03.2020 18:54





Advanced Placement (AP), 29.03.2020 18:54

Mathematics, 29.03.2020 18:54

French, 29.03.2020 18:54

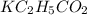 is, 24.5 grams
is, 24.5 grams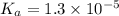
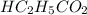 = 1.30 M
= 1.30 M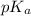 .
.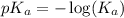
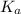 in this expression, we get:
in this expression, we get: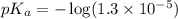
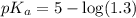
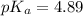
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0513/2213/e961a.png)
![pH=pK_a+\log \frac{[KC_2H_5CO_2]}{[HC_2H_5CO_2]}](/tpl/images/0513/2213/845a9.png)
![5.02=4.89+\log (\frac{[KC_2H_5CO_2]}{1.30})](/tpl/images/0513/2213/18fd8.png)
![[KC_2H_5CO_2]=1.75M](/tpl/images/0513/2213/46461.png)