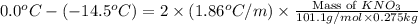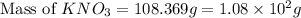Chemistry, 17.02.2020 17:59 Joshuafranklindude
Assuming complete dissociation of the solute, how many grams of KNO3 must be added to 275 mL of water to produce a solution that freezes at −14.5 ∘C? The freezing point for pure water is 0.0 ∘C and Kf is equal to 1.86 ∘C/m .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2


Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
Assuming complete dissociation of the solute, how many grams of KNO3 must be added to 275 mL of wate...
Questions


Mathematics, 26.11.2020 04:30


History, 26.11.2020 04:30



Chemistry, 26.11.2020 04:30

History, 26.11.2020 04:30



Mathematics, 26.11.2020 04:30

Chemistry, 26.11.2020 04:30

Biology, 26.11.2020 04:30


Mathematics, 26.11.2020 04:30


Spanish, 26.11.2020 04:30

Mathematics, 26.11.2020 04:30

History, 26.11.2020 04:30

Biology, 26.11.2020 04:30

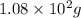
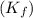 for water =
for water = 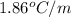
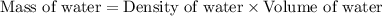
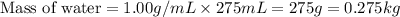
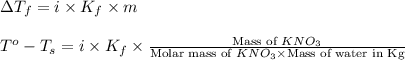
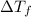 = change in freezing point
= change in freezing point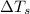 = freezing point of solution =
= freezing point of solution = 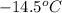
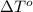 = freezing point of water =
= freezing point of water = 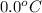
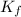 = freezing point constant for water =
= freezing point constant for water =