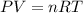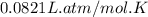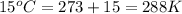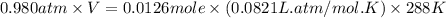Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
You know the right answer?
What volume of F2 (in liters) is required to react with 1.00 g of uranium according to the equation...
Questions

English, 23.03.2020 20:36

Mathematics, 23.03.2020 20:36

History, 23.03.2020 20:36

History, 23.03.2020 20:36





Mathematics, 23.03.2020 20:37


Biology, 23.03.2020 20:37

Mathematics, 23.03.2020 20:37


Social Studies, 23.03.2020 20:37

Biology, 23.03.2020 20:37


Mathematics, 23.03.2020 20:37


Mathematics, 23.03.2020 20:37

Mathematics, 23.03.2020 20:37

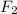 required is, 0.304 L
required is, 0.304 L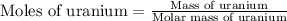
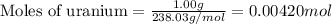
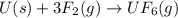
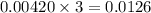 moles of
moles of