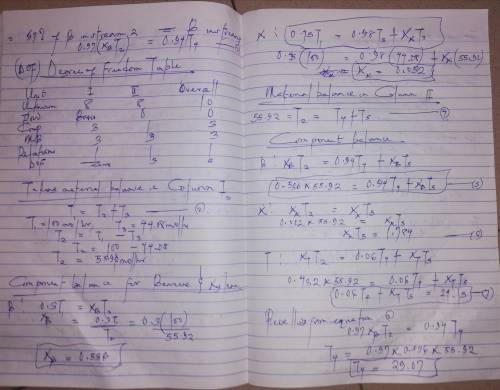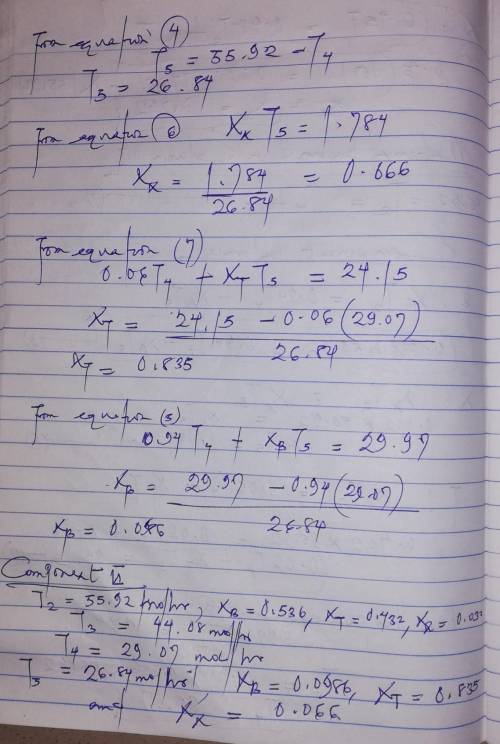Chemistry, 15.02.2020 04:23 lbabineaux9887
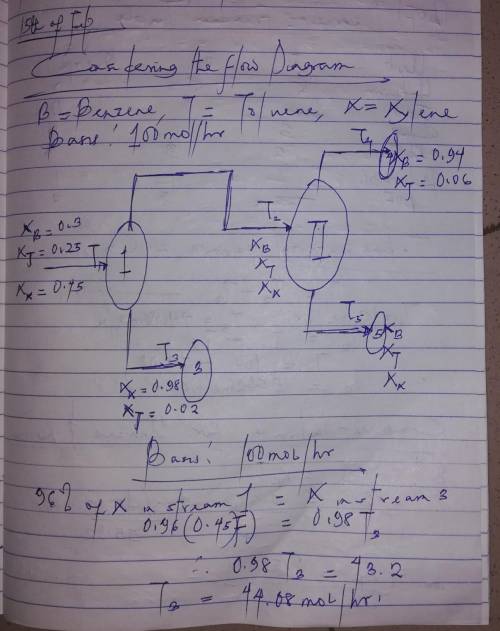
A liquid mixture containing 30.0 mole% benzene (B), 25.0% toluene (T), and the balance xylene (X) is fed to a distillation column. The bottoms product contains 98.0% X and no B, and 96.0% of the X in the feed is recovered in this stream. The overhead product is fed to a second column. The overhead product from the second column contains 97.0% of the B in the feed to this stream. The composition of this stream is 94.0 mole% B and the balance T. Draw and label a flowchart of this process and do the degree-of-freedom analysis to prove that for an assumed basis of calculation, molar flow rates and compositions of all process streams can be calculated from the given information. Write in order the equations you would solve to unknown process variables. In each equation (or pair of simultaneous equations), circle the variable(s) for which you would solve.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 07:20
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
A liquid mixture containing 30.0 mole% benzene (B), 25.0% toluene (T), and the balance xylene (X) is...
Questions




Biology, 25.08.2019 10:30

Biology, 25.08.2019 10:30

Biology, 25.08.2019 10:30

Mathematics, 25.08.2019 10:30

English, 25.08.2019 10:30


Mathematics, 25.08.2019 10:50



Mathematics, 25.08.2019 10:50


Social Studies, 25.08.2019 10:50

History, 25.08.2019 10:50