
Chemistry, 15.02.2020 04:27 emilymariec4036
The electron geometry and the molecular geometry of ammonia (NH3) are, respectively: The electron geometry and the molecular geometry of ammonia (NH3) are, respectively: tetrahedral, trigonal pyramidal. tetrahedral, tetrahedral. trigonal planar, bent. tetrahedral, bent. none of the above g

Answers: 2


Another question on Chemistry



Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1

Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
The electron geometry and the molecular geometry of ammonia (NH3) are, respectively: The electron ge...
Questions


Mathematics, 25.04.2021 14:00


Mathematics, 25.04.2021 14:00


Biology, 25.04.2021 14:00



English, 25.04.2021 14:00

Physics, 25.04.2021 14:00

Mathematics, 25.04.2021 14:00


Biology, 25.04.2021 14:00

English, 25.04.2021 14:00

Chemistry, 25.04.2021 14:00




Geography, 25.04.2021 14:00

![\frac{1}{2}[V+N-C+A]](/tpl/images/0512/2895/0d710.png)
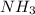
![\frac{1}{2}[5+3-0+0]=4](/tpl/images/0512/2895/ebbe0.png)
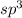 but as only 3 atoms are present , one position will be occupied by a lone pair thus electronic geometry is tetrahedral and the molecular geometry will be trigonal pyramidal.
but as only 3 atoms are present , one position will be occupied by a lone pair thus electronic geometry is tetrahedral and the molecular geometry will be trigonal pyramidal.

