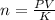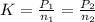Chemistry, 15.02.2020 04:26 viicborella
When carbonating fruit, the initial partial pressure of CO 2 is 4.00 x 10 – 4 atm, resulting in a concentration of 1.36 x 10 – 5 M . If the partial pressure of CO 2 rises to 0.25 atm, what is the new concentration of CO 2 dissolved?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1
You know the right answer?
When carbonating fruit, the initial partial pressure of CO 2 is 4.00 x 10 – 4 atm, resulting in a co...
Questions

Mathematics, 27.01.2021 05:20

Chemistry, 27.01.2021 05:20



Mathematics, 27.01.2021 05:20


Advanced Placement (AP), 27.01.2021 05:20

Biology, 27.01.2021 05:20




History, 27.01.2021 05:30

Mathematics, 27.01.2021 05:30

Mathematics, 27.01.2021 05:30

Mathematics, 27.01.2021 05:30

Mathematics, 27.01.2021 05:30

Biology, 27.01.2021 05:30

Chemistry, 27.01.2021 05:30


History, 27.01.2021 05:30