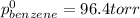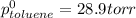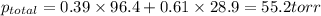Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1
You know the right answer?
Benzene has a formula of C6H6 and a vapor pressure of 96.4 torr at 298 K. Toluene has a formula of C...
Questions

Mathematics, 01.07.2020 20:01

Mathematics, 01.07.2020 20:01

Mathematics, 01.07.2020 20:01


Mathematics, 01.07.2020 20:01


Mathematics, 01.07.2020 20:01

Biology, 01.07.2020 20:01


Mathematics, 01.07.2020 20:01

Biology, 01.07.2020 20:01

History, 01.07.2020 20:01


History, 01.07.2020 20:01

Mathematics, 01.07.2020 20:01

English, 01.07.2020 20:01



Mathematics, 01.07.2020 20:01

History, 01.07.2020 20:01

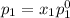 and
and 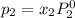
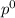 = pressure in the pure state
= pressure in the pure state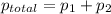
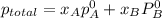
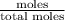
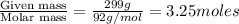
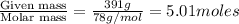
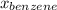 = mole fraction of benzene =
= mole fraction of benzene =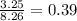
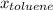 =mole fraction of toluene = (1-0.39) = 0.61
=mole fraction of toluene = (1-0.39) = 0.61