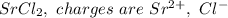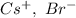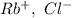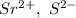Chemistry, 14.02.2020 19:38 alicemareus
The charges and sizes of the ions in an ionic compound affect the strength of the electrostatic attraction holding that compound together. Based on ion charges and relative ion sizes, rank these ionic compounds by their expected melting points from highest to lowest
a. SrCl2 ,b. CsBr ,c. RbCl ,d. SrS

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1


You know the right answer?
The charges and sizes of the ions in an ionic compound affect the strength of the electrostatic attr...
Questions

History, 30.03.2021 14:00

Health, 30.03.2021 14:00

Biology, 30.03.2021 14:00

Business, 30.03.2021 14:00


Mathematics, 30.03.2021 14:00

English, 30.03.2021 14:00

Mathematics, 30.03.2021 14:00

Chemistry, 30.03.2021 14:00

English, 30.03.2021 14:00

English, 30.03.2021 14:00


Mathematics, 30.03.2021 14:00


Mathematics, 30.03.2021 14:00

Mathematics, 30.03.2021 14:00

History, 30.03.2021 14:00

Engineering, 30.03.2021 14:00

Mathematics, 30.03.2021 14:00

History, 30.03.2021 14:00

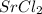 > RbCl > CsBr
> RbCl > CsBr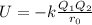
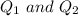 are charges and
are charges and 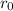 is the size.
is the size.