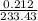Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1


Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
A solution contains an unknown mass of dissolved barium ions. When sodium sulfate is added to the so...
Questions

Mathematics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10




Mathematics, 18.03.2021 01:10

Spanish, 18.03.2021 01:10

Biology, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

Spanish, 18.03.2021 01:10

Computers and Technology, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10

Chemistry, 18.03.2021 01:10

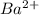 and
and 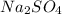 are added then white precipitate forms. And, reaction equation for this is as follows.
are added then white precipitate forms. And, reaction equation for this is as follows.
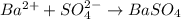
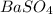 is 233.43.
is 233.43.