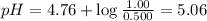Chemistry, 14.02.2020 03:28 andydiaz1227
What is the pH of a solution that is 0.500 M in acetic acid and 1.00 M in CH3COONa? Ka = 1.75*10-5 Group of answer choices 4.47 5.06 4.77 0.3 Flag this Question

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1


Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
What is the pH of a solution that is 0.500 M in acetic acid and 1.00 M in CH3COONa? Ka = 1.75*10-5 G...
Questions

Social Studies, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

English, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 03:01

Mathematics, 16.09.2020 03:01

Mathematics, 16.09.2020 03:01

Mathematics, 16.09.2020 03:01

Spanish, 16.09.2020 03:01

History, 16.09.2020 03:01

Mathematics, 16.09.2020 03:01

Mathematics, 16.09.2020 03:01

Mathematics, 16.09.2020 03:01

Mathematics, 16.09.2020 03:01

Mathematics, 16.09.2020 03:01

Mathematics, 16.09.2020 03:01

Mathematics, 16.09.2020 03:01

Mathematics, 16.09.2020 03:01

Mathematics, 16.09.2020 03:01