
Chemistry, 14.02.2020 01:41 katwright1124
Consider the following system at equilibrium where H° = -111 kJ, and Kc = 0.159 , at 723 K: N2 (g) + 3 H2 (g) 2 NH3 (g)
If the TEMPERATURE on the equilibrium system is suddenly decreased :
The value of Kc A. Increases B. Decreases C. Remains the same
The value of QcA. Is greater than Kc B. Is equal to Kc C. Is less than Kc
The reaction must: A. Run in the forward direction to restablish equilibrium. B. Run in the reverse direction to restablish equilibrium. C. Remain the same. Already at equilibrium.
The concentration of H2 will: A. Increase. B. Decrease. C. Remain the same.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2

Chemistry, 23.06.2019 11:00
Intermolecular forces. question i need with: the only intermolecular forces that affect non polar molecules are forces.
Answers: 2
You know the right answer?
Consider the following system at equilibrium where H° = -111 kJ, and Kc = 0.159 , at 723 K: N2 (g) +...
Questions

Mathematics, 22.01.2021 21:20



Arts, 22.01.2021 21:20




Engineering, 22.01.2021 21:20

Mathematics, 22.01.2021 21:20

Mathematics, 22.01.2021 21:20


English, 22.01.2021 21:20

Biology, 22.01.2021 21:20


Social Studies, 22.01.2021 21:20

Geography, 22.01.2021 21:20

Mathematics, 22.01.2021 21:20

Mathematics, 22.01.2021 21:20


English, 22.01.2021 21:20

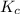 will increase.
will increase.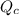 will decrease.
will decrease.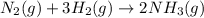
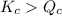 ( Move in froward direction )
( Move in froward direction )

