Chemistry, 14.02.2020 01:04 arthkk0877
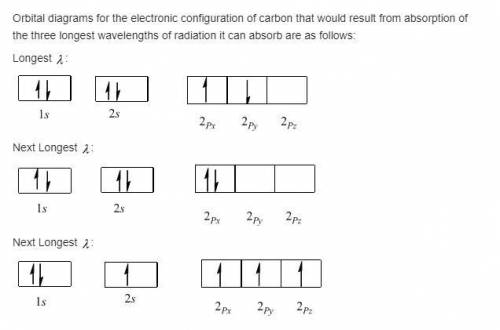
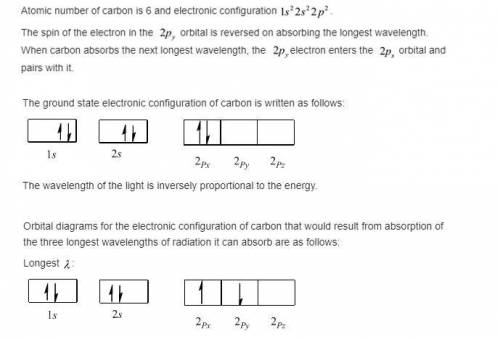
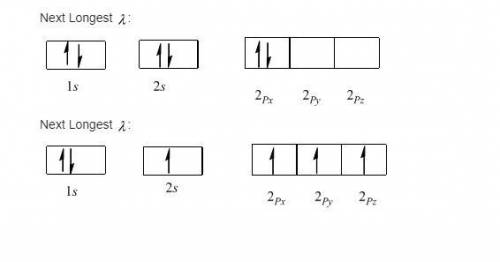
A carbon atom can absorb radiation of various wavelengths with resulting changes in its electronic configuration. Choose orbital diagrams for the electronic configurations of carbon that result from absorption of the three longest wavelengths of radiation that change its electronic configuration.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
A carbon atom can absorb radiation of various wavelengths with resulting changes in its electronic c...
Questions

Chemistry, 25.09.2021 14:00

History, 25.09.2021 14:00

History, 25.09.2021 14:00

Advanced Placement (AP), 25.09.2021 14:00



Biology, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00



Mathematics, 25.09.2021 14:00


Biology, 25.09.2021 14:00

English, 25.09.2021 14:00

English, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00



Mathematics, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00