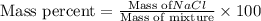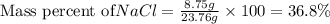Chemistry, 14.02.2020 00:16 alondrahernande3
Using the following data, determine the percent composition (by mass) of the NaCl (salt) in a binary mixture. The mass of the unknown binary mixture is 23.76g and the mass of the SiO2 (sand) alone is 15.01g. Record your answer to 3 significant figures, and include the percentage sign ('%'), or 'percent'.

Answers: 2


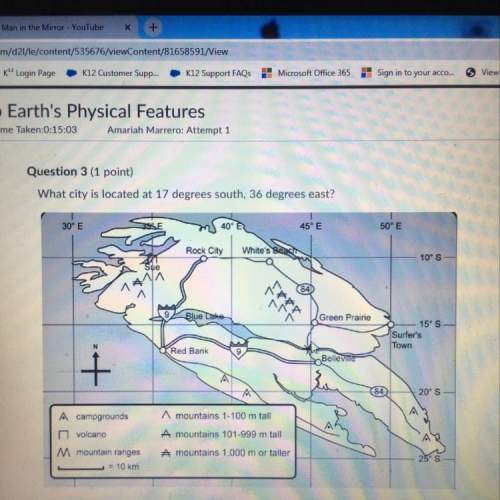
Another question on Chemistry


Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1
You know the right answer?
Using the following data, determine the percent composition (by mass) of the NaCl (salt) in a binary...
Questions


English, 06.11.2019 20:31

Mathematics, 06.11.2019 20:31


Mathematics, 06.11.2019 20:31




Biology, 06.11.2019 20:31


Biology, 06.11.2019 20:31


English, 06.11.2019 20:31

Social Studies, 06.11.2019 20:31

Mathematics, 06.11.2019 20:31

History, 06.11.2019 20:31


Mathematics, 06.11.2019 20:31

Physics, 06.11.2019 20:31

English, 06.11.2019 20:31

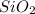 +Mass of
+Mass of 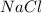 = 23.76 g
= 23.76 g