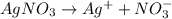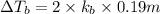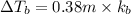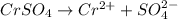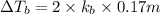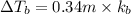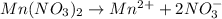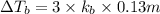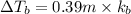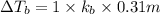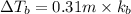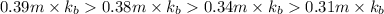Match the following aqueous solutions with the appropriate letter from the column on the right.1. 0.19 m AgNO3 2. 0.17 m CrSO4 3. 0.13 m Mn(NO3)2 4. 0.31 m Sucrose(nonelectrolyte) A. Highest boiling pointB. Second highest boiling pointC. Third highest boiling point D. Lowest boiling point

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 06:10
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1

Chemistry, 23.06.2019 10:30
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh.the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3

Chemistry, 23.06.2019 10:30
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 3.75 mol fe and 8.70 mol nio(oh) react?
Answers: 1

Chemistry, 23.06.2019 10:30
Me soon im confused much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 1
You know the right answer?
Match the following aqueous solutions with the appropriate letter from the column on the right.1. 0....
Questions



History, 15.01.2020 10:31


English, 15.01.2020 10:31

Biology, 15.01.2020 10:31

Chemistry, 15.01.2020 10:31

Mathematics, 15.01.2020 10:31

Mathematics, 15.01.2020 10:31





Chemistry, 15.01.2020 10:31


English, 15.01.2020 10:31


English, 15.01.2020 10:31

Business, 15.01.2020 10:31

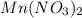 → Highest boiling point
→ Highest boiling point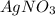 → Second Highest boiling point
→ Second Highest boiling point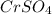 → Third highest boiling point
→ Third highest boiling point 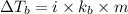
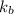 = Molal Elevation constant of solvent
= Molal Elevation constant of solvent