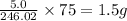Chemistry, 13.02.2020 20:54 golderhadashaowtatz
The mineral orpiment, having the empirical formula As2S3, was used in ancient times as a cosmetic. What mass of arsenic is present in 5.0 g of orpiment? Hint: Determine the percent composition of As in As2S3 , then take that percent of 5.0 g.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
The mineral orpiment, having the empirical formula As2S3, was used in ancient times as a cosmetic. W...
Questions


Mathematics, 05.01.2021 22:50

Mathematics, 05.01.2021 22:50

Mathematics, 05.01.2021 22:50



Mathematics, 05.01.2021 22:50

Mathematics, 05.01.2021 22:50

Spanish, 05.01.2021 22:50


Mathematics, 05.01.2021 22:50

Mathematics, 05.01.2021 22:50



Mathematics, 05.01.2021 22:50

Mathematics, 05.01.2021 22:50

Mathematics, 05.01.2021 22:50

Mathematics, 05.01.2021 22:50

Social Studies, 05.01.2021 22:50

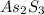 is, 246.02 g/mol and the molar mass of As is, 75 g/mol.
is, 246.02 g/mol and the molar mass of As is, 75 g/mol.