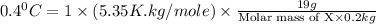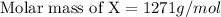Chemistry, 13.02.2020 18:46 kingdrex4772
When 19g of a certain molecular compound X are dissolved in of benzonitrile , the freezing point of the solution is measured to be . Calculate the molar mass of X.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Apackage contains 1.33 lb of ground round. if there’s 29% fat, how many grams of fat are in the round? i got 175 g but the textbook says the answer is 91 g of fat. how?
Answers: 2

Chemistry, 21.06.2019 15:00
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3
You know the right answer?
When 19g of a certain molecular compound X are dissolved in of benzonitrile , the freezing point of...
Questions

Arts, 29.03.2021 18:50


Mathematics, 29.03.2021 18:50



Mathematics, 29.03.2021 18:50

English, 29.03.2021 18:50

Chemistry, 29.03.2021 18:50

Social Studies, 29.03.2021 18:50







Mathematics, 29.03.2021 18:50

Mathematics, 29.03.2021 18:50



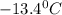 . Calculate the molar mass of X. Freezing point of pure benzonitrile =
. Calculate the molar mass of X. Freezing point of pure benzonitrile = 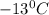
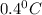
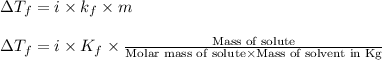
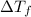 = change in freezing point =
= change in freezing point = 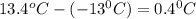
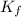 = freezing point constant = 5.35 K.kg/mole
= freezing point constant = 5.35 K.kg/mole