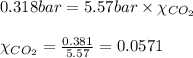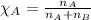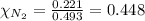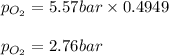Chemistry, 13.02.2020 18:57 AJSkullcrusher
A student has a 2.19 L bottle that contains a mixture of O 2 , N 2 , and CO 2 with a total pressure of 5.57 bar at 298 K . She knows that the mixture contains 0.221 mol N 2 and that the partial pressure of CO 2 is 0.318 bar . Calculate the partial pressure of O 2.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3


Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
A student has a 2.19 L bottle that contains a mixture of O 2 , N 2 , and CO 2 with a total pressure...
Questions

History, 11.10.2019 08:31






History, 11.10.2019 08:31

Mathematics, 11.10.2019 08:31

English, 11.10.2019 08:31


History, 11.10.2019 08:31


Mathematics, 11.10.2019 08:31

Mathematics, 11.10.2019 08:31

Mathematics, 11.10.2019 08:31



Chemistry, 11.10.2019 08:31

Chemistry, 11.10.2019 08:31

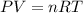
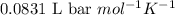
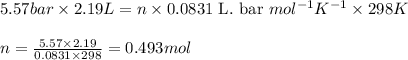
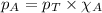 ........(1)
........(1)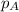 = partial pressure of carbon dioxide = 0.318 bar
= partial pressure of carbon dioxide = 0.318 bar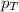 = total pressure = 5.57 bar
= total pressure = 5.57 bar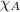 = mole fraction of carbon dioxide = ?
= mole fraction of carbon dioxide = ?