

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
Using the systematic approach for equilibrium problems, calculate the pH of 0.05 M HOCl. Ka= 3.0*10-...
Questions



Social Studies, 14.12.2020 19:40

Mathematics, 14.12.2020 19:40

Mathematics, 14.12.2020 19:40



Mathematics, 14.12.2020 19:40


Mathematics, 14.12.2020 19:40

Mathematics, 14.12.2020 19:40


Mathematics, 14.12.2020 19:40

Mathematics, 14.12.2020 19:40

English, 14.12.2020 19:40

Mathematics, 14.12.2020 19:40

Mathematics, 14.12.2020 19:40

French, 14.12.2020 19:40


French, 14.12.2020 19:40

![Ka=\frac{[ClO-]*[H+]}{[HClO]}=\frac{x*x}{0.05-x}=3x10^{-8}](/tpl/images/0510/1394/ee229.png)
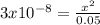
![x=3.87x10^{-5}=[H+]=[ClO-]](/tpl/images/0510/1394/d0c35.png)
![pH=-log[H+]=-log[3.87x10^{-5}]=4.41](/tpl/images/0510/1394/b0e39.png)


