
Chemistry, 13.02.2020 05:55 jasonoliva13
Which of the following sets are isoelectronic (i. e., have the same number of electrons)? i. Br-, Kr, Sr2+ ii. C, N-, O2- iii. Mg2+, Ca2+, Sr2+ iv. O2-, O, O2+ v. Ag+, Cd2+, Pd

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
Which of the following sets are isoelectronic (i. e., have the same number of electrons)? i. Br-, Kr...
Questions

Mathematics, 02.03.2021 06:00

Biology, 02.03.2021 06:00


Mathematics, 02.03.2021 06:00

Geography, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00

Biology, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00

Law, 02.03.2021 06:00


Biology, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00

History, 02.03.2021 06:00

Social Studies, 02.03.2021 06:00

English, 02.03.2021 06:00

Social Studies, 02.03.2021 06:00


Mathematics, 02.03.2021 06:00

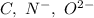
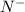 has 6 electrons.
has 6 electrons.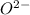 has 6 electrons.
has 6 electrons.

