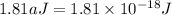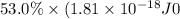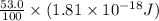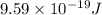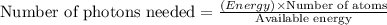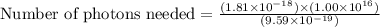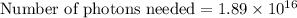Chemistry, 13.02.2020 04:09 blakeley7785
Assuming an ionization efficiency of 53.0 % , how many such photons are needed to ionize 1.00 × 10 16 atoms?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
Assuming an ionization efficiency of 53.0 % , how many such photons are needed to ionize 1.00 × 10 1...
Questions


Mathematics, 02.05.2021 02:20


History, 02.05.2021 02:20


Mathematics, 02.05.2021 02:20












Mathematics, 02.05.2021 02:20

English, 02.05.2021 02:20