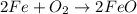A student reacts steel wool with oxygen by touching the fibers with a 9 volt battery. They begin
with 7.93 g Fe and measure the mass of the final product at 9.50 g.
The balanced equation for the reaction is:
2 Fe + 02 --> 2 FeO
A) The student claims that the percent yield of the reaction was 93.1 %. Support or reject their
claim including a calculation of percent yield as part of your evidence.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1
You know the right answer?
A student reacts steel wool with oxygen by touching the fibers with a 9 volt battery. They begin
Questions






Mathematics, 10.09.2019 04:10


English, 10.09.2019 04:10



Spanish, 10.09.2019 04:10









Computers and Technology, 10.09.2019 04:10