
Chemistry, 12.02.2020 23:28 purplepig12
If the freezing point of the solution had been incorrectly read 0.3 °C lower than the true freezing point, would the calculated molar mass of the solute have been too high or too low? Explain.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
If the freezing point of the solution had been incorrectly read 0.3 °C lower than the true freezing...
Questions


Mathematics, 18.12.2020 18:40

Mathematics, 18.12.2020 18:40



Mathematics, 18.12.2020 18:40


Mathematics, 18.12.2020 18:40






Arts, 18.12.2020 18:40

Mathematics, 18.12.2020 18:40


Arts, 18.12.2020 18:40

History, 18.12.2020 18:40

History, 18.12.2020 18:40

History, 18.12.2020 18:40

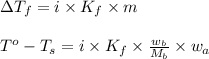
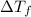 = change in freezing point
= change in freezing point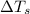 = freezing point of solution
= freezing point of solution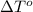 = freezing point of water
= freezing point of water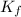 = freezing point constant
= freezing point constant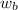 = mass of solute
= mass of solute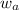 = mass of solvent
= mass of solvent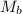 = molar mass of solute
= molar mass of solute

