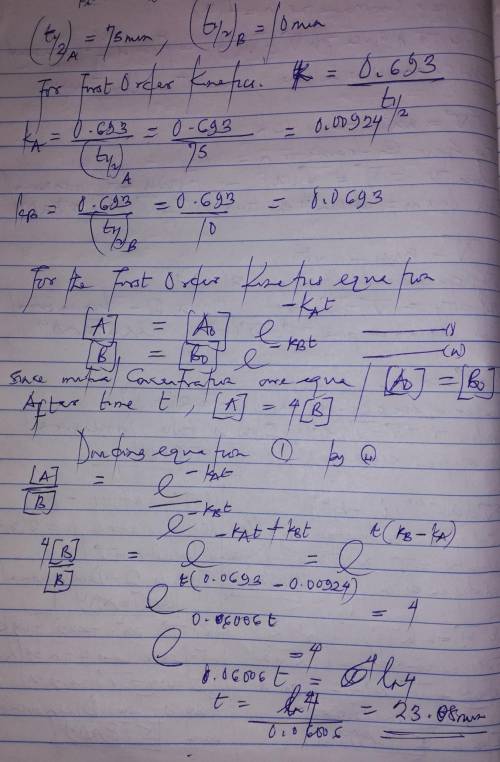Chemistry, 12.02.2020 22:50 sadieruegner393
A flask contains a mixture of compounds A and B. Both compounds decompose by first-order kinetics. The half-lives are 75.00 min for A and 10.00 min for B. If the concentrations of A and B are equal initially, how long will it take for the concentration of A to be four times that of B

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

You know the right answer?
A flask contains a mixture of compounds A and B. Both compounds decompose by first-order kinetics. T...
Questions


Law, 25.09.2020 23:01

Mathematics, 25.09.2020 23:01

History, 25.09.2020 23:01

Mathematics, 25.09.2020 23:01



English, 25.09.2020 23:01


Computers and Technology, 25.09.2020 23:01


Geography, 25.09.2020 23:01





History, 25.09.2020 23:01

History, 25.09.2020 23:01

History, 25.09.2020 23:01