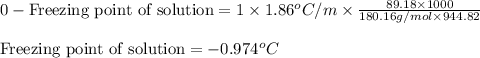Chemistry, 12.02.2020 18:31 gracebuffum
At 298 K, the osmotic pressure of a glucose solution (C6H12O6 (aq)) is 12.1 atm. Calculate the freezing point of the solution. The density of the solution is 1.034 g/mL.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 23.06.2019 04:20
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2

Chemistry, 23.06.2019 06:40
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1
You know the right answer?
At 298 K, the osmotic pressure of a glucose solution (C6H12O6 (aq)) is 12.1 atm. Calculate the freez...
Questions





Biology, 12.11.2020 17:20


Mathematics, 12.11.2020 17:20

Social Studies, 12.11.2020 17:20








History, 12.11.2020 17:20


Mathematics, 12.11.2020 17:20


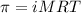
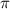 = osmotic pressure of the solution = 12.1 atm
= osmotic pressure of the solution = 12.1 atm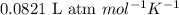
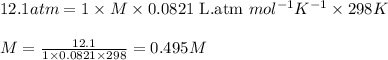
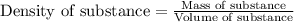
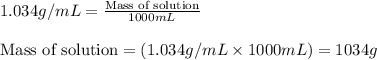
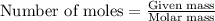
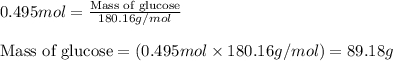
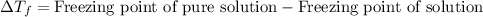
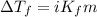
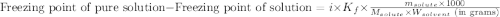
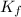 = molal freezing point elevation constant = 1.86°C/m
= molal freezing point elevation constant = 1.86°C/m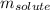 = Given mass of solute (glucose) = 89.18 g
= Given mass of solute (glucose) = 89.18 g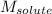 = Molar mass of solute (glucose) = 180.16 g/mol
= Molar mass of solute (glucose) = 180.16 g/mol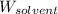 = Mass of solvent (water) = [1034 - 89.18] g = 944.82 g
= Mass of solvent (water) = [1034 - 89.18] g = 944.82 g