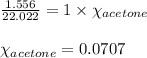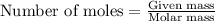Chemistry, 12.02.2020 05:58 mjlchance367
When a specific amount of acetone (C3H6O) is added to 100.0 g of pure water at 65°C, the vapor pressure of water over the solution is lowered by 1.556 kPa. Given the vapor pressure of water at 65°C is 25.022 kPa, what is the mass of acetone added?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
When a specific amount of acetone (C3H6O) is added to 100.0 g of pure water at 65°C, the vapor press...
Questions




Social Studies, 22.03.2021 18:30

Mathematics, 22.03.2021 18:30

Mathematics, 22.03.2021 18:30

Mathematics, 22.03.2021 18:30


Mathematics, 22.03.2021 18:30

History, 22.03.2021 18:30


Mathematics, 22.03.2021 18:30




Mathematics, 22.03.2021 18:30

Health, 22.03.2021 18:30

Mathematics, 22.03.2021 18:30


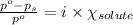
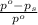 = relative lowering in vapor pressure = 1.556 kPa
= relative lowering in vapor pressure = 1.556 kPa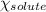 = mole fraction of solute = ?
= mole fraction of solute = ?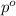 = vapor pressure of pure water = 22.022 kPa
= vapor pressure of pure water = 22.022 kPa