
Chemistry, 12.02.2020 05:48 aliveajones2005
A 5.00-mole sample of NH3 gas is kept in a 1.92-L container at 300 K. If the van der Waals equation is assumed for the pressure of the gas, calculate the percent error made in using the ideal-gas equation to calculate the pressure.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2


Chemistry, 23.06.2019 11:00
Just on number 2 (all parts), and if you do answer explain in detail
Answers: 3
You know the right answer?
A 5.00-mole sample of NH3 gas is kept in a 1.92-L container at 300 K. If the van der Waals equation...
Questions

English, 24.02.2021 14:00



Mathematics, 24.02.2021 14:00



Mathematics, 24.02.2021 14:00

Chemistry, 24.02.2021 14:00

Mathematics, 24.02.2021 14:00

Mathematics, 24.02.2021 14:00

Social Studies, 24.02.2021 14:00

Mathematics, 24.02.2021 14:00


Geography, 24.02.2021 14:00

Mathematics, 24.02.2021 14:00



Mathematics, 24.02.2021 14:00

English, 24.02.2021 14:00

English, 24.02.2021 14:00

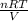
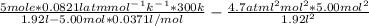
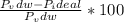 %
%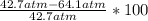 %
%

