
Chemistry, 12.02.2020 05:28 friendsalwaysbae
For a ternary solution at constant T and P, the composition dependence of molar property M is given by: M = x1M1 + x2M2 + x3M3 + x1 x2 x3C where M1, M2, and M3 are the values of M for pure species 1, 2, and 3, and C is a parameter independent of composition. Determine expressions for M¯1,M¯2, and M¯3 by application of Eq. (10.7). As a partial check on your results, verify that they satisfy the summability relation, Eq. (10.11). For this correlating equation, what are the M¯i at infinite dilution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3
You know the right answer?
For a ternary solution at constant T and P, the composition dependence of molar property M is given...
Questions

Engineering, 01.12.2020 07:20






Mathematics, 01.12.2020 07:20

Mathematics, 01.12.2020 07:20


Mathematics, 01.12.2020 07:20


English, 01.12.2020 07:20



History, 01.12.2020 07:20

Health, 01.12.2020 07:20

Advanced Placement (AP), 01.12.2020 07:20

Mathematics, 01.12.2020 07:20

Mathematics, 01.12.2020 07:20

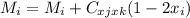 ...1
...1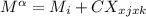 ...2
...2![M_{i} = [\frac{d(nM)}{dn_{i} }]_{P,t,n,j}](/tpl/images/0508/2556/4b4ae.png)


