
Chemistry, 12.02.2020 05:30 pxrnstar4613
Which of the following statements about Avogadro’s Law is false? a. One mole of He occupies 22.4 L at STP. b. At constant P and T, volume and moles of gas are directly proportional. c. At constant T and P, doubling the moles of gas decreases the volume by half. d. At the same T and P, 2 mol of gas occupies twice the volume of one mole of gas. e. An equal number of moles of different gases occupy the same volume at STP.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:50
Your roll: experienced electron speech is adressed to: a new "freshman class" of electrons job: write a speech task: you are to pretend that you are giving a speech to a new group of electrons. be sure to mention their placement in an atom, their charge, and their role in chemical bonding (ionic and covalent) be specific!
Answers: 3

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2
You know the right answer?
Which of the following statements about Avogadro’s Law is false? a. One mole of He occupies 22.4 L a...
Questions



Biology, 15.10.2019 14:10


English, 15.10.2019 14:10









Biology, 15.10.2019 14:10






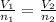 where
where

