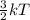A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon. Sort the conditions based on the gas described. Some may have more than one answer, but noe will have the same answer.
1) Nitrogen has the greater value
2) Xenon has the greater value
3) Both gases have the same value
A. Gas that exerts the greater partial pressure.
B. Gas for which the molecules or atoms have the greatest average velocity
C. Gas that would have a higher rate of effusion through a small hole opened in the flask.
D. Gas for which the molecules or atoms have the greatest average of kinetic energy.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1


Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon. Sort th...
Questions

Mathematics, 09.06.2021 06:30


Mathematics, 09.06.2021 06:30


Mathematics, 09.06.2021 06:40

Mathematics, 09.06.2021 06:40


Mathematics, 09.06.2021 06:40



Health, 09.06.2021 06:40



Mathematics, 09.06.2021 06:40

Advanced Placement (AP), 09.06.2021 06:40

Geography, 09.06.2021 06:40


Mathematics, 09.06.2021 06:40