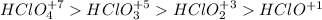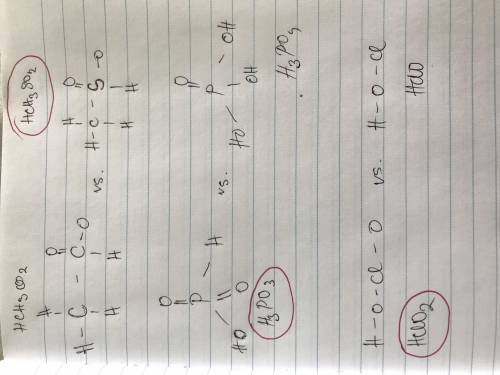Chemistry, 12.02.2020 04:49 jerseygirl1783
In each row, check the box under the compound that can reasonably be expected to be more acidic in aqueous solution, e. g. have the larger Ka
a) HCH3CO2 vs HCH3SO2
b) H3PO4 vs H3PO3
c HClO2 vs HClO
Please explain why.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2
You know the right answer?
In each row, check the box under the compound that can reasonably be expected to be more acidic in a...
Questions

Mathematics, 02.08.2019 05:30



English, 02.08.2019 05:30

Social Studies, 02.08.2019 05:30


Mathematics, 02.08.2019 05:30

History, 02.08.2019 05:30

Biology, 02.08.2019 05:30

Health, 02.08.2019 05:30

History, 02.08.2019 05:30

Mathematics, 02.08.2019 05:30


Social Studies, 02.08.2019 05:30

Social Studies, 02.08.2019 05:30


Mathematics, 02.08.2019 05:30



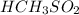
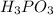
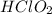
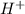 ) and anion (
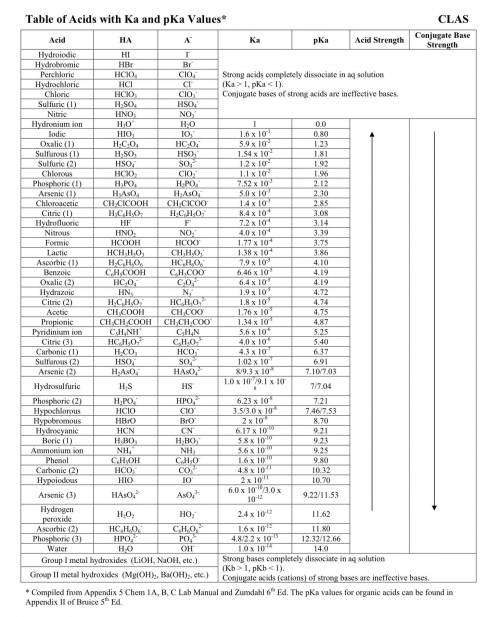
) and anion (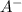 ) in aqueous solution. Acid strength can be determined by measuring this tendency to separate into proton an anion. Strength of an acid can be quantified by its acid dissociation value - Ka. A strong acid will have a tendency to easily release proton and will have larger Ka value and smaller logarithmic value (pKa = - logKa) similar to calculating pH of the solution. So the easiest way to resolve this issue is by looking for Ka or pKa value of the acid (This table may be useful in more complex tasks and is attached below). However, stronger acid can be determined elsehow.
) in aqueous solution. Acid strength can be determined by measuring this tendency to separate into proton an anion. Strength of an acid can be quantified by its acid dissociation value - Ka. A strong acid will have a tendency to easily release proton and will have larger Ka value and smaller logarithmic value (pKa = - logKa) similar to calculating pH of the solution. So the easiest way to resolve this issue is by looking for Ka or pKa value of the acid (This table may be useful in more complex tasks and is attached below). However, stronger acid can be determined elsehow. 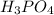 , phosphorus holds one double bond with oxygen and three OH group equally. To show an acidic tendency, phosphorus would need to let go one hydrogen out of one of OH groups. In
, phosphorus holds one double bond with oxygen and three OH group equally. To show an acidic tendency, phosphorus would need to let go one hydrogen out of one of OH groups. In