Consider the reaction
H2(g) + 1 2 O2(g) → H2O(ℓ) + 286 kJ
How much H2 would...

Chemistry, 12.02.2020 04:42 gabrielbergemancat
Consider the reaction
H2(g) + 1 2 O2(g) → H2O(ℓ) + 286 kJ
How much H2 would have to be burned to yield America’s daily energy share of 260,000 kcal? (1 cal = 4.184 J)
1.) 1900 mol
2.) 3800 mol
3.) 7600 mol
4.) 1 mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2
You know the right answer?
Questions




English, 25.02.2021 01:00




Mathematics, 25.02.2021 01:00

Chemistry, 25.02.2021 01:00







Biology, 25.02.2021 01:00

Mathematics, 25.02.2021 01:00

English, 25.02.2021 01:00


English, 25.02.2021 01:00

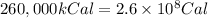 (Conversion factor: 1 kCal = 1000 Cal)
(Conversion factor: 1 kCal = 1000 Cal)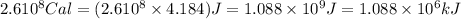
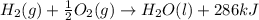
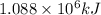 of energy will be released when
of energy will be released when 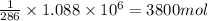 of hydrogen gas is consumed
of hydrogen gas is consumed

