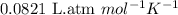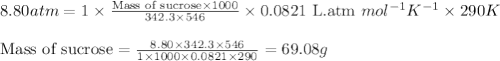Chemistry, 12.02.2020 03:24 cuppykittyy
What mass of sucrose (C12H22O11) should be combined with 546 g of water to make a solution with an osmotic pressure of 8.80 atm at 290 K ? (Assume the density of the solution to be equal to the density of the solvent.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1


Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
You know the right answer?
What mass of sucrose (C12H22O11) should be combined with 546 g of water to make a solution with an o...
Questions








Spanish, 11.09.2019 04:30

Physics, 11.09.2019 04:30

Mathematics, 11.09.2019 04:30

Mathematics, 11.09.2019 04:30





Mathematics, 11.09.2019 04:30

Mathematics, 11.09.2019 04:30

Social Studies, 11.09.2019 04:30

Mathematics, 11.09.2019 04:30

Mathematics, 11.09.2019 04:30

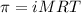
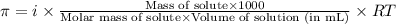
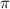 = osmotic pressure of the solution = 8.80 atm
= osmotic pressure of the solution = 8.80 atm