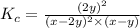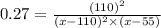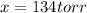Chemistry, 12.02.2020 02:45 ghaithalhamdani
Nitric oxide reacts with chlorine gas according to the following reaction:
2NO(g)+Cl2(g)?2NOCl(g)
Kp=0.27 at 700 K
A reaction mixture initially contains equal partial pressures of NO and Cl2. At equilibrium, the partial pressure of NOCl was measured to be 110 torr. What were the initial partial pressures of NO and Cl2?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3

Chemistry, 23.06.2019 05:30
Find the midpoint of a segment with endpoints of 4-3i and -2+7i
Answers: 2

Chemistry, 23.06.2019 10:00
State the effect on the concentration of the clo- ion when there is a decrease in the concentration of the oh- ion
Answers: 1
You know the right answer?
Nitric oxide reacts with chlorine gas according to the following reaction:
2NO(g)+Cl2(g)?2NOC...
2NO(g)+Cl2(g)?2NOC...
Questions

Social Studies, 23.07.2019 15:40

Biology, 23.07.2019 15:40

Mathematics, 23.07.2019 15:40

Advanced Placement (AP), 23.07.2019 15:40





Physics, 23.07.2019 15:40


Social Studies, 23.07.2019 15:40

History, 23.07.2019 15:40

Biology, 23.07.2019 15:40

Biology, 23.07.2019 15:40

History, 23.07.2019 15:40

Social Studies, 23.07.2019 15:40

Mathematics, 23.07.2019 15:40

Business, 23.07.2019 15:40

Social Studies, 23.07.2019 15:40

Business, 23.07.2019 15:40

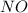 and
and 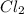 are 134 torr each.
are 134 torr each.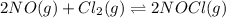
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0507/8534/56950.png)