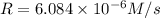The initial rate data at 25 oC are listed for the reaction
NH4+ (aq) + NO2-1 -> N2(g) + H2O(l)
Experiment Initial [NH4+1] Initial [NO2-1] Initial rate (M/s)
1 0.24 0.1 7.2 X10^-6
2 0.12 0.1 3.6 X 10^-6
3 0.12 0.15 5.4 X 10^-6
a. Determine the rate law
b. Determine the value of the rate constant.
c. What is the reaction rate when the concentrations are [NH4+1] = 0.39 M and [NO2-1] = 0.052 M.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
The initial rate data at 25 oC are listed for the reaction
NH4+ (aq) + NO2-1 -> N2(g)...
NH4+ (aq) + NO2-1 -> N2(g)...
Questions

Mathematics, 10.01.2021 19:10


Mathematics, 10.01.2021 19:10


Biology, 10.01.2021 19:10









Mathematics, 10.01.2021 19:10



Mathematics, 10.01.2021 19:10

Advanced Placement (AP), 10.01.2021 19:10

Mathematics, 10.01.2021 19:10

![=k[NH_4^{+}]^1[NO_2^{-}]^1](/tpl/images/0507/8247/fe0cf.png)
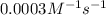
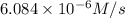 is the reaction rate when the concentrations are
is the reaction rate when the concentrations are ![[NH_4^{+}]](/tpl/images/0507/8247/7aa39.png) = 0.39 M and
= 0.39 M and ![[NO_2^{-}]](/tpl/images/0507/8247/f8e12.png) = 0.052 M.
= 0.052 M.![=k[NH_4^{+}]^x[NO_2^{-}]^y](/tpl/images/0507/8247/8a532.png)
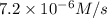
![R=k[0.24 M]^x[0.1 M]^y](/tpl/images/0507/8247/5fc35.png) ...[1]
...[1]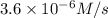
![R'=k[0.12 M]^x[0.1 M]^y](/tpl/images/0507/8247/447c7.png) ...[2]
...[2]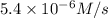
![R''=k[0.12 M]^x[0.15 M]^y](/tpl/images/0507/8247/eedcf.png) ...[3]
...[3]![\frac{R}{R'}=\frac{k[0.24 M]^x[0.1]^y}{k[0.12 M]^x[0.1]^y}](/tpl/images/0507/8247/5b2fa.png)
![\frac{7.2\times 10^{-6} M/s}{3.6\times 10^{-6} M/s}=\frac{k[0.24 M]^x[0.1 M]^y}{k[0.12 M]^x[0.1 M]^y}](/tpl/images/0507/8247/5d06d.png)
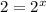
![\frac{R'}{R''}=\frac{k[0.12 M]^x[0.1 M]^y}{k[0.12 M]^x[0.15]^y}](/tpl/images/0507/8247/52502.png)
![\frac{3.6\times 10^{-6} M/s}{5.4\times 10^{-6} M/s}=\frac{k[0.12 M]^x[0.1 M]^y}{k[0.12 M]^x[0.15 M]^y}](/tpl/images/0507/8247/db163.png)
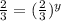
![7.2\times 10^{-6} M/s=k[0.24 M]^1[0.1 M]^1](/tpl/images/0507/8247/29f30.png) ...[1]
...[1]![k=\frac{7.2\times 10^{-6} M/s}{[0.24 M]^1[0.1 M]^1}=0.0003 M^{-1} s^{-1}](/tpl/images/0507/8247/38560.png)
![R=0.0003 M^{-1} s^{-1}\times [0.39 M][0.052 M]](/tpl/images/0507/8247/2f5df.png)