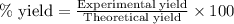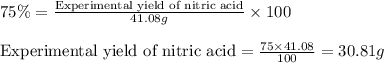Chemistry, 12.02.2020 01:38 jennemylesp19oy5
If the percent yield for the following reaction is 75.0%, and 45.0 g of NO2 are consumed in the reaction, how many grams of nitric acid, HNO3(aq), are produced?
3 NO2(g) + H2O(l) ? 2 HNO3(aq) + NO(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1


Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3
You know the right answer?
If the percent yield for the following reaction is 75.0%, and 45.0 g of NO2 are consumed in the reac...
Questions


Mathematics, 07.11.2020 08:20


English, 07.11.2020 08:20

Health, 07.11.2020 08:20






Chemistry, 07.11.2020 08:20


Mathematics, 07.11.2020 08:20

History, 07.11.2020 08:20

Mathematics, 07.11.2020 08:20

Chemistry, 07.11.2020 08:20

Mathematics, 07.11.2020 08:20

Mathematics, 07.11.2020 08:20

Mathematics, 07.11.2020 08:20

Arts, 07.11.2020 08:20

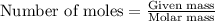 .....(1)
.....(1)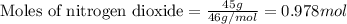
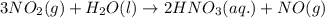
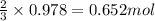 of nitric acid
of nitric acid